Project B3 | Oligoanionic inositol phosphates as therapeutics
Keller / Castagner
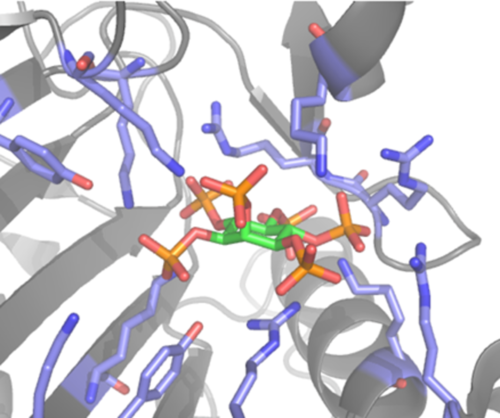
Here we will address the therapeutic potential of small molecule polyelectrolytes. Inositol hexakisphosphate (IP6) contains six phosphate groups, which are partially deprotonated under physiological conditions. This highly charged molecule is a cofactor for a number of endogenous proteins, as well as bacterial toxins such as TcdA and TcdB of Clostridium difficile and viral proteins such as Gag from HIV-1. In a competing reaction, IP6 forms a complex with divalent cations, such as Ca2+ and Mg2+, which reduces the concentration of free IP6 to the point that IP6 is ineffective in the presence of Ca2+. Modulating the physico-chemical properties of inositol phosphate polyanion has promising therapeutic potential.
Favouring the interaction of inositol phosphates with TcdB while reducing calcium chelation is a novel therapeutic approach against the pathogen C. difficile. Replacing some of the phosphate groups by sulfate groups leads to reduced calcium chelation, while retaining interaction with TcdB. In a C. difficile infection mouse model this lead to considerably reduced mortality. Interestingly, not only the total number of sulfate groups but also their position along the inositol ring determined the efficacy of the IP6 analogues. Additionally, it is known that TcdB undergoes a major structural transition from the inactive to the active form of the protein upon binding ofP6. This allosteric transition can possibly be modified by targeting a neighbouring binding pocket. In this project, we aim to better understand the detailed molecular interactions between the oligoanionic IP6 and TcdB using modelling and molecular-dynamics simulation in order to inform the design of next-generation drug candidates. Here, we propose to investigate the equilibrium constant of the calcium chelate complexes and of the IP6-analogue-protein complexes as a function of Ca2+-concentration, temperature and pH in silico as well as by isothermal titration calorimetry measurements. Additionally, the project aims to improve the specificity of the IP6 analogues by linking them to small fragments that are putative binders of the neighbouring pocket. These fragments will be linked in silico to IP6 analogues via various linking strategy and docked into the proteins, and the flexibility of the substrate within the binding pocket will be investigated by short molecular-dynamics simulations. The best analogues will then be synthesized, structurally characterized together with A2 and evaluated further. The in-depth understanding of the competition between chelate-complex formation and the oligoanion-protein-complex formation as well as simulation and modelling will allow us to rapidly design superior drug candidates.
The molecular simulations will be carried out at Freie Universität Berlin. The project involves modelling of charged molecules, including force-parameterization, free energy calculations and the analysis of correlations in complex biomolecules.
Subjects: Chemistry | Biochemistry | Theory