Project C2 | Polyanions for targeting inflammation in skin and adipose tissue
Achazi / Dernedde / Conway / Hedtrich
Polyanionic dendritic polyglycerol sulfates (dPGS) target different proteins linked to inflammation due to electrostatic interactions. When compared to the natural, anti-inflammatory, linear polysulfate heparin, most dPGS effects are more distinct and the different architecture might contribute to the respective outcome. In project C2 the highly cationic chemokine, platelet factor 4 (PF4) will be evaluated as a potential target for dPGS. Structural changes of PF4, will be analyzed with spectroscopic methods together with A3 while the kinetics and thermodynamics of binding will be evaluated using surface plasmon resonance as well as ITC with A1.
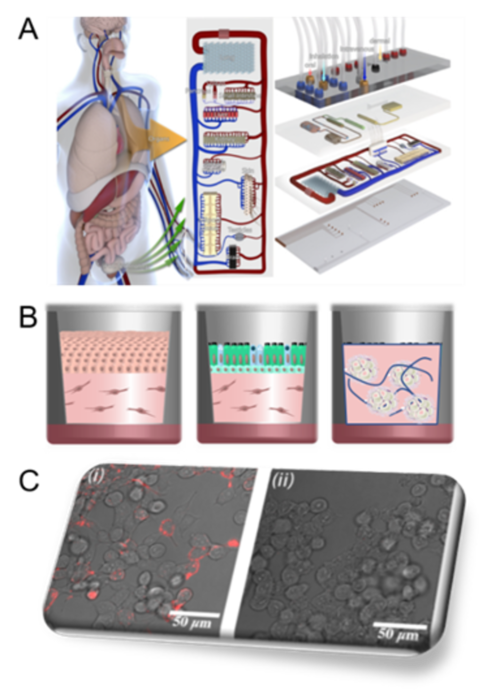
PF4 is released into the circulation primarily from platelets, whereupon it binds to vascular proteoglycans and other polyanions. For example, there is strong evidence that PF4 plays a central role in allergic diseases, such as atopic dermatitis (AD) and allergic asthma (AA). Indeed, PF4 is significantly upregulated in AD and AA patients, with levels that are directly correlated with disease severity. In AA, PF4 appears to drive airway inflammation. Nevertheless, although links have been described, little is known about the mechanistic contributions of PF4 and its potential as a therapeutic target. Moreover, its role in other inflammatory disorders has not been fully explored; nor have means to intervene in its pro-inflammatory activities. In project C2 we will investigate the role of PF4 in the context of exogenous dPGS, in models of two common inflammatory disorders. The first are allergic human-based disease models that emulate characteristics of inflammatory skin diseases and the bronchial epithelium in an organ-on-a-chip setup. These will be used to determine the potential of dPGS to bind PF4 in a diseased state to alleviate potential pro-inflammatory effects. Live-cell imaging in combination with super resolution microscopy will be used to follow the fate of compounds by labelling them with imaging probes. Furthermore, applicability of polysulfates as competitive binders to glycosaminoglycans will be analyzed. The second model is an in vitro 3D spheroid model of adipose tissue, generated from primary human or murine adipose-derived preadipocytes that can recapitulate the pathophysiologic changes that occur in the chronic inflammation of obesity. This approach optimizes cell-cell and cell-extracellular matrix interactions in the absence of a scaffold. In conjunction with live-cell imaging to monitor the fate of the compounds added during spheroid growth/differentiation, we will monitor the effects of the compounds on insulin sensitivity, insulin signalling pathways, response to lipogenic stresses, and the release of adipocytokines, growth factors and inflammatory mediators
Subjects: Biochemistry | Pharmacology