Project C1 | Polyelectrolyte blood and glycocalyx interactions
Weinhart / Kizhakkedathu
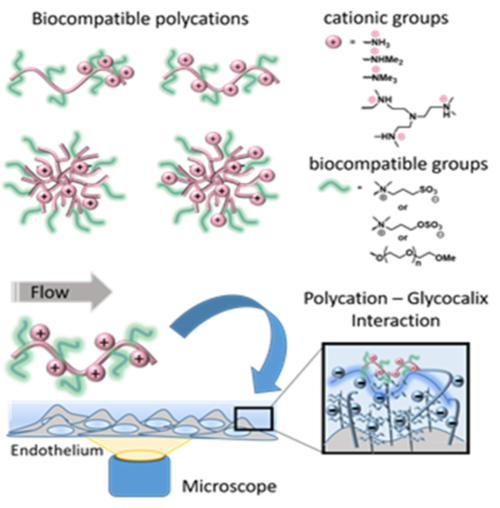
The luminal side of blood vessel walls is lined by a complex, 0.5-5 µm thick dynamic hydrogel layer composed of membrane bound or adsorbed and network forming proteoglycans, glycosaminoglycans (GAGs), as well as glyco- and plasma proteins. Besides its function as a mechanotransducing barrier between the circulating blood components and the vessel wall, this endothelial glycocalyx has been increasingly recognized as an immune modulator and a therapeutic target in various disease such as atheriosclerosis, sepsis, vasculitis and transplantation in recent years. Due to the complex architecture and the dynamic assembly and enzymatic degradation, the network forming as well as cytokine, chemokine and plasma protein binding interactions within the glycocalyx are still not fully understood. However, a better understanding of these interactions within the glycocalyx is crucial to fully utilize the potential of the endothelial glycocalyx as a therapeutic target.
Within this project, a cell-based, dynamically cultured in vitro model of the endothelial glycocalyx with adjustable composition, thickness, hydration, and charge density will be established to study electrostatic interactions with synthetic polymers and blood cells. The polymers serve as readily available and structurally adjustable models for relevant cytokines and proteins, such as HMGB-1 or PF4. The often-encountered intrinsic cellular toxicity of synthetic polycations is prevented by the presentation of biocompatible hydrophilic oligo ethylene glycol (OEG) or zwitterionic groups on the polymer structure which do not hinder the electrostatic binding to blood components or GAGs. The binding events on the endothelial glycocalyx will be traced and investigated microscopically by fluorescent colocalization in a flow chamber set-up. Adjustable shear stress will be applied in the presence or absence of competing electrostatic binding partners while the soluble GAG content in plasma from shedding events is quantified for correlation with the binding events. Activation states of the endothelium will be measured using antibodies targeting. Glycocalyx shedding as shown in disease conditions and its influence on polycation binding will be modelled by activating the endothelium using TNFa. Complementary force microscopy measurements will yield semiquantitative data on the glycocalyx interactions with blood cells. The picture will be completed by functional blood or plasma assays applying the same model polycations in solution, and its binding to blood based polyanions such as polyphosphate, extracellular nucleic acids and GAGs.
Subjects: Chemistry | Macromolecular Chemistry | Biophysics