Project A3 | Elucidating structural implications of polyelectrolyte protein interactions
Risse / Srebnik / Multhaup
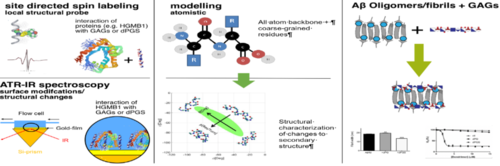
Structure and function of proteins are strongly correlated, which renders knowledge about protein structure an important prerequisite for an atomistic understanding of their properties. This project aims at elucidating the impact of the interaction between proteins and polyelectrolytes on their structure by combining experimental and theoretical studies. The project will focus on two systems namely the interaction of HMGB1 (High-Mobility-Group-Protein B1) with polyelectrolytes focusing in particular on polysulfates such as GAGs and the interaction of oligomers as well as fibrils of Aβ42 with polyelectrolytes.
On the method side, site directed spin labelling (SDSL)/EPR spectroscopy will be combined with surface enhanced IR spectroscopy to obtain information on the fold of the investigated proteins in presence of polyelectrolytes. Furthermore, surface plasmon resonance as well as quartz crystal microbalance studies will provide access to the kinetics of the corresponding adsorption/desorption processes. The experimental work is complemented by theoretical studies, which will employ molecular models to simulate the dynamics involved in the secondary and tertiary structural changes of the protein in the presence of polyelectrolytes.
Subjects: Chemistry | Biochemistry | Biophysics | Theory