Project A2 | Glycosaminoglycans and their interaction with amyloid-forming peptides
Pagel / Koksch / Multhaup
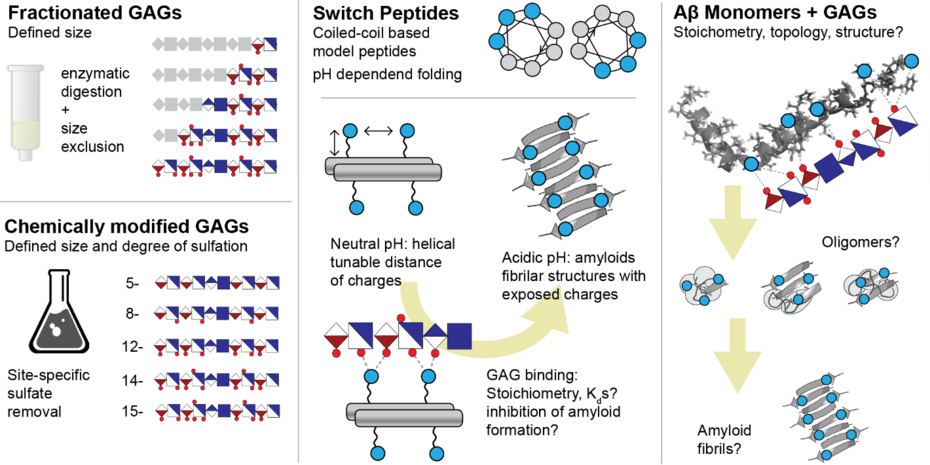
Glycosaminoglycans (GAGs) are negatively charged polysaccharides, which are characterized by a linear sequence of repeating disaccharide units that are densely sulfated. Being present in the extracellular matrix and at the surface of cells, they regulate various biological processes by binding to peptides, proteins and their complexes. The most prominent example is heparin, which is used as anticoagulant in hospitals since the 1930s. GAG-protein binding is to a large extent governed by multivalent interaction between positive charges at the surface of the protein and negative charges located at hexuronic acid and sulfate moieties within the GAG. However, due to the lack of synthetic standards and the inability to efficiently isolate pure GAGs from biological material, to date very little is known about the molecular details and thermodynamics of GAG-protein interactions.
A particularly interesting but also underexplored aspect is the binding of GAGs to amyloid forming peptides. Amyloid formation is the hallmark of many neurodegenerative diseases such as Alzheimer’s and Parkinson. A common feature in all these diseases is the assembly of monomeric, often partial helical peptides into large, insoluble, beta-sheet-rich aggregates. It is known that amyloid peptides such as Ab can bind to GAGs, however, the resulting consequences for the assembly process and the toxicity of the formed aggregates are not understood. Aim of this project is to elucidate the binding of GAGs to small amyloidogenic peptides.
The Pagel group will use a combination of enzymatic digestion as well as size exclusion and anion exchange chromatography to purify highly sulphated GAG fragments from commercially available material such as heparin. Simultaneously, switch peptides – i.e. synthetic model peptides that undergo a structural transition from small helical assemblies into amyloids upon changes of the environmental conditions – will be designed, synthesized and purified in the Koksch group. In these peptides, the density and distance of positive charges can be controlled by design, which will enable a systematic analysis of GAG-binding on the molecular level. In parallel, the Multhaup lab will prepare various truncated Aβ variants that have been detected in AD brains. These samples will be incubated with GAG fragments and studied regarding their assembly characteristics. Special focus will be the impact of GAGs on the structure of the helical Aβ monomers as well as the toxic early oligomers (dimers and tetramers). The obtained data will yield fundamentally new insights how the aggregation of Aβ is affected by the presence of GAGs in the extracellular matrix.
Subjects: Chemistry | Biochemistry | Analytical Chemistry